What Is UDI? FDA's Unique Device Identification System Guide 2025
- Beng Ee Lim

- Jul 29, 2025
- 6 min read
Updated: Sep 7, 2025
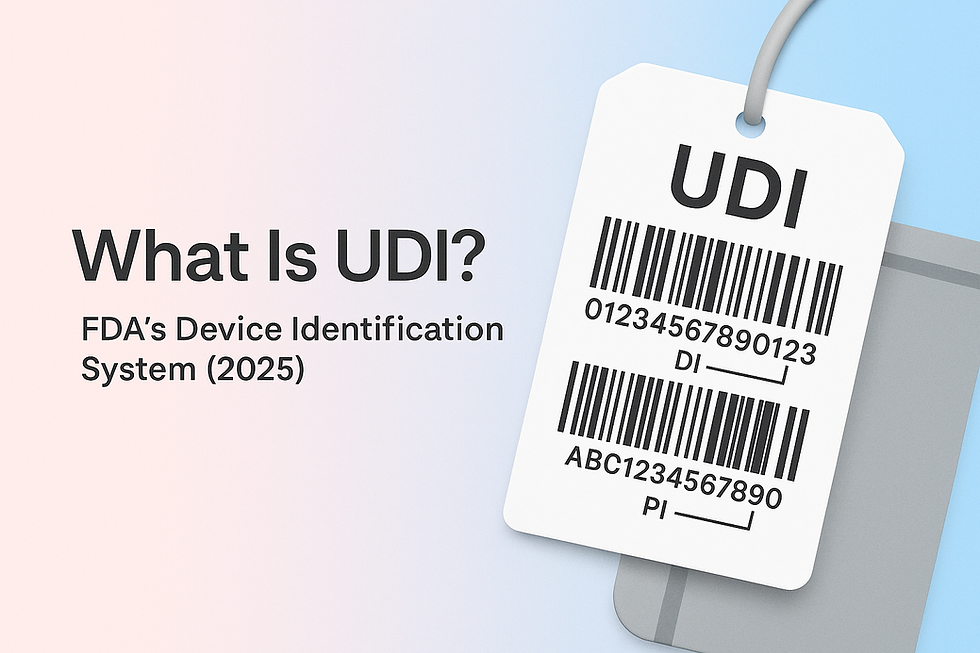
UDI is FDA’s Unique Device Identification system: most device labels and packages must carry a UDI in machine-readable (AIDC) and human-readable (HRI) form; certain reusable, reprocessed devices also require direct marking. Each UDI contains a fixed Device Identifier (DI) and variable Production Identifier (PI); DI records (not PI values) are submitted to GUDID.
What Is UDI and Why It Matters for Your Medical Device
The Unique Device Identification (UDI) system is FDA's comprehensive framework for identifying and tracking medical devices from manufacturing through distribution to patient use. The UDI Rule requires device labelers to include a unique device identifier on device labels and packages, and submit device information to the Global Unique Device Identification Database (GUDID).
Here's what makes UDI critical: Every medical device entering the U.S. market must carry a UDI unless specifically exempted. This isn't optional—it's a legal requirement that affects labeling, manufacturing, quality systems, and market timeline.
The compliance landscape is unforgiving. Devices labeled on or after September 24, 2023, must comply with all applicable UDI requirements, including prohibition on legacy FDA identification numbers. Companies still using outdated systems face immediate compliance issues.
How UDI Components Work Together
Device Identifier (DI)
The DI is your device's permanent "fingerprint"—a fixed code that identifies:
Device labeler (typically the manufacturer)
Specific version or model of the device
Package configuration for that model
Key fact: The DI remains constant for each device version and serves as the key to obtain device information in GUDID.
Production Identifier (PI)
The PI captures variable production information when included on labels:
Lot or batch number within which device was manufactured
Serial number of the device
Manufacturing date of the device
Expiration date if applicable
Distinct identification code for certain regulated products
Critical distinction: The FDA does not require PI data in GUDID submissions, though labelers must indicate which PI elements are present on the label.
What Devices Need UDI Compliance
All Class II and III Devices
Every Class II & III device—sterile or non-sterile—must now display a UDI and have its DI uploaded to GUDID. Direct-mark grace periods ended 2015–2018 (check FDA’s “Compliance Policies & Dates” table for edge-case alternatives).
Class I Device Requirements
The Class I landscape is complex:
CGMP-exempt Class I devices: Excepted from UDI requirements under 21 CFR 801.30
Consumer health products: FDA does not intend to enforce GUDID submission requirements for Class I devices considered consumer health products that are required to bear a UDI
Implantable/life-supporting Class I: Must comply with full UDI requirements
Key Exemptions
Your device is exempt from UDI if it's:
Custom device within meaning of 21 CFR 812.3(b)
Investigational device under IDE
Used solely for research, teaching, or chemical analysis
Intended for export from the United States
Veterinary device not intended for human use
GUDID: The Global Database You Must Master
What Is GUDID
GUDID (Global Unique Device Identification Database) is a digital catalog created by FDA to store detailed information on all medical devices with a unique device identifier. Think of it as the central registry where your device's "birth certificate" lives.
GUDID Submission Requirements
Who submits: Device labelers are responsible for GUDID submissions, which in most instances would be the device manufacturer, but may be a specification developer, reprocessor, convenience kit assembler, repackager, or relabeler.
What you submit: There are 57 attributes which GUDID requires for submissions, broken down into categories including device identifier information, commercial distribution, alternative identifiers, customer contact details, manufacturing information, and sterilization specifications.
Two Submission Methods
Manual entry: GUDID web application for entering data for one device at a time
Bulk upload: HL7 SPL submission via FDA Electronic Submissions Gateway for submitting multiple records simultaneously
When UDI Compliance Kicks In
Current Status: Implementation Complete
The UDI system is being implemented in phases, and we are currently in the final phase of implementation which includes the lowest risk devices.
Critical 2023 Deadline Impact
September 24, 2023 marked a major transition: Devices labeled on or after September 24, 2023, must comply with all applicable UDI requirements, including prohibition on legacy FDA identification numbers (NDC and NHRIC numbers).
What this means: If you're still using old NDC or NHRIC numbers on labels, you're already non-compliant. The transition period has ended.
Direct Marking Requirements
Reusable devices: If a device is intended for more than one use and intended to be reprocessed before each use, the device labeler must also mark the UDI directly on the device.
Compliance policy: FDA does not intend to enforce direct mark requirements for devices manufactured and labeled prior to their applicable direct mark compliance date that remain in inventory, provided the device bears a non-UDI direct mark.
Common UDI Mistakes That Cost Companies Thousands
Labeling Errors
Barcode sizing mistakes: Common mistake: design for the wrong dpi - you risk creating a label that doesn't match the printer's correct dots per inch resolution
Edge printing problems: Barcodes can stretch over the edge simply because a value changes from numeric to alphanumeric
GUDID Submission Failures
Data quality issues: UDI data may be stored in different locations (siloed databases, file systems, local computers), may not be accurate or current, stored in varying formats, and have numerous data owners
Missing preparation: Complete required steps before requesting a GUDID account including reviewing UDI guidance documents, working with FDA-accredited issuing agencies, and gathering data required for GUDID DI records
Issuing Agency Selection Problems
Wrong choice costs time and money: Manufacturers must obtain UDI numbers only from FDA-accredited issuing agencies. Most companies choose GS1, but ICCBBA exists for specific medical products of human origin.
Step-by-Step UDI Implementation Process
Phase 1: Assessment and Planning (Month 1)
Determine device classification and UDI requirements
Review current labeling for compliance gaps
Assess GUDID data availability across systems
Select FDA-accredited issuing agency (typically GS1)
Phase 2: System Setup (Months 2-3)
Establish issuing agency account and obtain company prefix
Request GUDID account with appropriate user roles
Develop standard operating procedures for UDI assignment
Create label templates meeting FDA requirements
Phase 3: Data Preparation (Month 4)
Assign Device Identifiers to each device version/model
Compile GUDID submission data for all 57 required attributes
Validate data accuracy across all sources
Prepare production identifier protocols for manufacturing
Phase 4: Implementation (Month 5)
Submit initial GUDID entries using chosen method
Update labeling and packaging with UDI requirements
Implement direct marking for applicable devices
Train manufacturing staff on UDI processes
Phase 5: Ongoing Compliance (Ongoing)
Update GUDID data within required timeframes
Maintain UDI assignment procedures for new devices
Monitor regulatory changes and guidance updates
Audit compliance regularly for continued adherence
UDI Cost Planning: Budget for Success
Initial Setup Costs
Issuing agency fees: GS1 charges initial fee plus annual renewal
GUDID account setup: Free from FDA
System integration: Variable based on complexity
Label redesign: Design and validation costs
Ongoing Operational Costs
Annual issuing agency fees: Typically hundreds to thousands annually
GUDID maintenance: Staff time for data updates
Label printing updates: New printing requirements
Compliance monitoring: Regular audit and review costs
Hidden Cost Avoidance
Prevent expensive mistakes: Non-compliance can result in warning letters, product seizures, or market delays. Non-compliance with UDI standards can lead to regulatory action including warnings, fines, product seizures, or injunctions against further production.
Global UDI Landscape: Beyond FDA Requirements
EU UDI Requirements
MDR placing UDI-carriers on device labels: Class III devices by May 26, 2021; Class IIa and IIb devices by May 26, 2023; Class I devices by May 26, 2025.
Other Markets Following Suit
Brazil: ANVISA released UDI regulation entering into force January 10, 2022, with a six-year rollout according to risk class
China: UDI requirements implemented with stepwise approach for different device classes
Global strategy needed: Companies selling internationally must navigate multiple UDI systems with varying requirements and timelines.
The Fastest Path to Market
No more guesswork. Move from research to a defendable FDA strategy, faster. Backed by FDA sources. Teams report 12 hours saved weekly.
FDA Product Code Finder, find your code in minutes.
510(k) Predicate Intelligence, see likely predicates with 510(k) links.
Risk and Recalls, scan MAUDE and recall patterns.
FDA Tests and Standards, map required tests from your code.
Regulatory Strategy Workspace, pull it into a defendable plan.
👉 Start free at complizen.ai

FAQ
Q: Do all medical devices need UDI?
A: No. Class I CGMP-exempt devices, custom devices, investigational devices, and devices for export are exempt. However, most commercially distributed devices require UDI.
Q: What happens if I don't comply with UDI requirements?
A: FDA can take enforcement action including warning letters, fines, product seizures, or injunctions. Non-compliant devices may need recalls.
Q: Can I use my existing product codes as UDI?
A: Only if issued by an FDA-accredited issuing agency like GS1. Legacy NDC and NHRIC numbers are no longer permitted on labels.
Q: How often must I update GUDID data?
A: Updates must be submitted no later than when a device is first labeled with changed information, or within 10 business days if the information doesn't appear on labels.
Q: What's the difference between UDI and GUDID?
A: UDI is the identifier on your device label. GUDID is the FDA database where you submit device information linked to that UDI.


