top of page

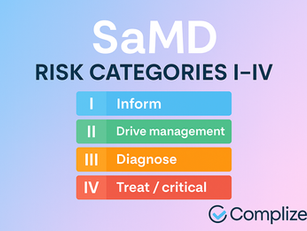
SaMD Risk Categories (IMDRF): What They Mean & How They Relate to FDA Review
IMDRF categorizes SaMD risk (I–IV) by crossing significance of information (inform, drive, treat/diagnose) with the condition’s...
May 13, 20253 min read


Device Software Function (DSF): FDA’s 2025 AI Draft Definition
“For purposes of this guidance, FDA refers to a software function that meets the definition of a device as a ‘device software function.’”...
May 8, 20253 min read


What Counts as SaMD? FDA Definition in Plain English
Software as a Medical Device (SaMD) = software that has a medical purpose and performs that purpose without being part of a hardware...
May 7, 20254 min read


FDA Unique Device Identification (UDI): Complete Guide for Medical Device Manufacturers
FDA’s UDI system requires most device labels and packages to bear a Unique Device Identifier —in human-readable and AIDC...
Dec 6, 20244 min read


FDA Medical Device Recalls Explained: Causes, Consequences, and How to Avoid Them
An FDA medical device recall is a firm-initiated correction or removal of a violative device, conducted voluntarily and monitored by FDA...
Dec 3, 20246 min read


FDA Form 483: What It Means and How to Respond Effectively
Form FDA 483 (Inspectional Observations) lists conditions FDA investigators observed at the end of an inspection. It is not a final...
Nov 29, 20244 min read
bottom of page
